CDC Immunization Guidelines
Introduction to CDC Immunization Guidelines
The Centers for Disease Control and Prevention (CDC) plays a crucial role in protecting public health and safety in the United States. One of the key areas of focus for the CDC is the development and dissemination of immunization guidelines. These guidelines are designed to provide healthcare professionals with the information they need to make informed decisions about vaccine administration and to help prevent the spread of infectious diseases. In this blog post, we will delve into the world of CDC immunization guidelines, exploring their importance, the process of development, and the key recommendations for various age groups and populations.
Importance of Immunization Guidelines
Immunization guidelines are essential for several reasons. Firstly, they help to ensure that individuals are protected against serious and potentially life-threatening diseases. Vaccines have been proven to be highly effective in preventing the spread of infectious diseases, and by following the CDC’s immunization guidelines, healthcare professionals can help to protect not only their patients but also the wider community. Secondly, immunization guidelines help to promote consistency and standardization in vaccine administration, reducing the risk of errors and adverse reactions. Finally, the guidelines provide a framework for healthcare professionals to stay up-to-date with the latest developments in vaccine research and technology, ensuring that they are equipped to provide the best possible care for their patients.
Process of Developing Immunization Guidelines
The CDC’s immunization guidelines are developed through a rigorous and transparent process. The process begins with the formation of a working group, comprising experts from various fields, including pediatrics, infectious diseases, and epidemiology. This working group reviews the latest scientific evidence and conducts thorough analyses of vaccine safety and efficacy. The group also considers factors such as vaccine availability, cost, and feasibility of implementation. Once the working group has completed its review, the draft guidelines are submitted to the Advisory Committee on Immunization Practices (ACIP) for review and approval. The ACIP is a panel of experts that provides advice to the CDC on immunization-related issues. After the ACIP has approved the guidelines, they are published and made available to healthcare professionals and the general public.
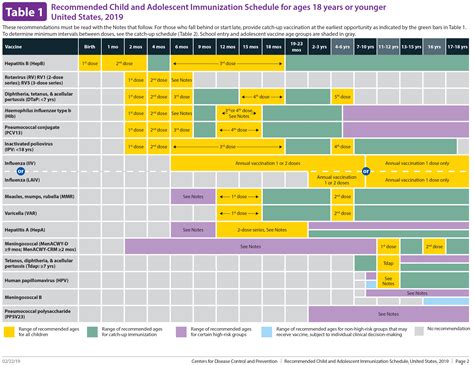
Key Recommendations for Various Age Groups and Populations
The CDC’s immunization guidelines provide recommendations for various age groups and populations. The guidelines are divided into several sections, including: * Children and Adolescents: The guidelines recommend a series of vaccines for children and adolescents, including vaccines against diseases such as measles, mumps, and rubella (MMR), diphtheria, tetanus, and pertussis (DTaP), and human papillomavirus (HPV). * Adults: The guidelines recommend vaccines for adults, including vaccines against diseases such as influenza, pneumococcal disease, and hepatitis A and B. * Pregnant Women: The guidelines recommend vaccines for pregnant women, including vaccines against diseases such as influenza and tetanus, diphtheria, and pertussis (Tdap). * Healthcare Workers: The guidelines recommend vaccines for healthcare workers, including vaccines against diseases such as influenza, hepatitis B, and meningococcal disease.
Vaccine Administration and Scheduling
The CDC’s immunization guidelines provide detailed information on vaccine administration and scheduling. The guidelines recommend the following: * Vaccine Administration: The guidelines provide information on the proper administration of vaccines, including the route of administration, dosage, and timing. * Vaccine Scheduling: The guidelines provide information on the recommended schedule for vaccine administration, including the age at which vaccines should be administered and the interval between doses.
| Vaccine | Age | Dosage |
|---|---|---|
| MMR | 12-15 months | 1 dose |
| DTaP | 2, 4, 6, and 15-18 months | 4 doses |
| HPV | 11-12 years | 2-3 doses |
📝 Note: The vaccine schedule and administration may vary depending on individual circumstances, such as medical history and risk factors.
Monitoring and Evaluation of Immunization Guidelines
The CDC continuously monitors and evaluates the effectiveness of its immunization guidelines. The agency collects data on vaccine coverage, disease incidence, and adverse events, and uses this information to update and refine the guidelines as necessary. The CDC also collaborates with other organizations, such as the World Health Organization (WHO) and the National Institutes of Health (NIH), to stay up-to-date with the latest developments in vaccine research and technology.
In summary, the CDC’s immunization guidelines play a critical role in protecting public health and safety in the United States. By following these guidelines, healthcare professionals can help to prevent the spread of infectious diseases and promote consistency and standardization in vaccine administration. The guidelines are developed through a rigorous and transparent process, and are continuously monitored and evaluated to ensure their effectiveness.
What is the purpose of the CDC's immunization guidelines?
+
The purpose of the CDC's immunization guidelines is to provide healthcare professionals with the information they need to make informed decisions about vaccine administration and to help prevent the spread of infectious diseases.
How are the CDC's immunization guidelines developed?
+
The CDC's immunization guidelines are developed through a rigorous and transparent process, involving the formation of a working group, review of the latest scientific evidence, and approval by the Advisory Committee on Immunization Practices (ACIP).
What vaccines are recommended for children and adolescents?
+
The CDC recommends a series of vaccines for children and adolescents, including vaccines against diseases such as measles, mumps, and rubella (MMR), diphtheria, tetanus, and pertussis (DTaP), and human papillomavirus (HPV).
In final consideration, the CDC’s immunization guidelines are a vital resource for healthcare professionals and the general public. By understanding the importance, development, and key recommendations of these guidelines, individuals can make informed decisions about vaccine administration and help to protect themselves and their communities from infectious diseases.